Anodized Aluminum:Process,Benefits,Design Tips
Dec 15, 2022
Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.
The process is called anodizing because the part to be treated forms the anode electrode of an electrolytic cell. Anodizing increases resistance to corrosion and wears and provides better adhesion for paint primers and glues than bare metal does. Anodic films can also be used for several cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add reflected light wave interference effects.
What metals can be anodized?
Aluminum, aluminum alloys, magnesium, titanium, and stainless steel can be anodized. Aluminum is by far the most common due to its high strength-to-weight ratio and availability. Anodized aluminum is capable of achieving many colors as dyes can be used to get the desired shade.
What is Anodized Aluminum?
Anodized aluminum is aluminum that has been surface treated to produce a highly durable finish. It is more aesthetically pleasing and has superior anti-corrosive properties. We can use an electrochemical process to make anodized aluminum. In this process, the metal is immersed in a series of tanks, wherein in one of the tanks, the anodic layer grows directly from the metal itself.
Because this anodized layer is formed from the aluminum itself, as opposed to being painted on or applied, anodized aluminum is impervious to chipping, flaking, and peeling and is more durable than any comparable material on the market. Aluminum that has been anodized is three times harder than the raw material and 60% lighter than competitive metals such as stainless steel and copper.
Anodized aluminum parts are the most widely used and valuable material in manufacturing thousands of industrial and household products. Despite having long-lasting effects on its properties, the anodizing process retains the natural appearance of aluminum.
How to Anodise Aluminum?
Before learning how to anodize aluminum, it is necessary to understand how anodizing works. The anodizing process appears complex because of the multiple electrochemical processes that occur. It is pretty easy and cost-effective. The following are the general steps in the anodizing process.
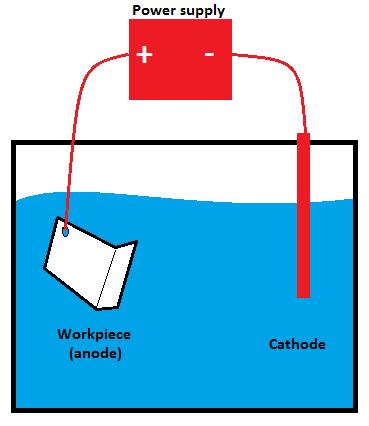
Step 1:Clean the aluminum metal and place it into an electrolyte solution.
The first step is properly cleaning the aluminum metal, which is then thoroughly rinsed. The aluminum is subsequently placed into an electrolyte solution primarily composed of sulfuric or chromic acid. An electrolyte solution is an electrically charged liquid containing several positive and negative ions that conduct the charge throughout the medium.
Step 2:Apply a direct electric current and create an electrochemical reaction.
When a positive charge is applied to aluminum, it serves as an anode, while the suspending plates in the solution act as a cathode when a negative charge is provided to them. In doing so, an electric circuit is formed in the electrolytic solution, which transfers current from a positive to a negative charge.
In anodizing, the positive ions flow toward the negatively charged plates as the current passes. During this period, oxygen ions are separated from the electrolyte acid and attached to the aluminum piece immersed in the electrolytic solution. The attachment of oxygen ions to aluminum results in a thick aluminum oxide layer, which can increase durability, improve appearance, and increase resistance.
Aluminum or any other metal anodizing can be modified by changing the nature of the electrolyte, the temperature of the setting, the voltage used to pass current, and the composition of the electrolytic solution.
What are the 3 Common Types of Anodizing Processes?
Based on their unique advantages and the electrolytic acid utilized, there are various types of aluminum anodizing processes. The common types are Type I-Chromic Acid Anodize, Type II-Sulfuric Acid Anodize, and Type III-Hardcoat Anodize (or Hard Anodize).
Type I Anodizing – Chromic Acid Anodizing
The Type I anodizing process employs chromic acid to form a thin coating on the surface of metal parts (up to 0.0001 inches). Even though Type I is the thinnest anodizing coating of the three principal types, it nonetheless increases the corrosion resistance of parts.
Type I appears considerably grayer and absorbs less color when dyed, which restricts the use of chromic acid anodize as a decorative finish. Nevertheless, chromic acid anodize can be dyed black for use as a non-reflective protective coating on optical component housings.Even black dyed chromic anodize seems lighter (grayer) than traditional sulfuric black anodize.
Chromic Acid Anodize Features:
-
Good for Bonding.
-
Non-Conductive.
-
It can be dyed black; other colors are not practical.
-
Suitable for parts with tight tolerances because it won’t change the size.
-
Suitable for welded parts and assemblies.
Chromic Acid Anodize Applications:
-
As a prime or paint base.
-
Aerospace Components.
-
Precision Machined Components.
-
Welded components and assemblies.
Type II Anodizing – Sulfuric Acid Anodizing
The most used method for anodizing is the sulfuric acid process. The sulfuric acid anodize produces films ranging in thickness from 0.0002 to 0.001 inches. The overall thickness of the produced coating is 67% penetration in the substrate and a 33% increase over the part’s initial dimension. It is ideally suited for applications that demand hardness and abrasion resistance.
When making colored finishes on aluminum and its alloys, the porous nature of sulfuric acid films before sealing has a particular advantage. The porous aluminum oxide absorbs dyes efficiently, and subsequent sealing helps to avoid color loss in service. Some colors include black, red, blue, green, urban gray, coyote brown, and gold.

Sulfuric Acid Anodize Features:
-
Harder than chromic acid anodize.
-
A clearer finish allows for more color options when it dyes.
-
Suited for applications that demand hardness and abrasion resistance.
-
Less expensive than other types of anodizing in terms of chemicals utilized, heating, energy consumption, and time required to achieve the desired thickness.
-
Waste Treatment is more straightforward than chromic anodizing, which also helps to reduce cost.
Sulfuric Acid Anodize Applications:
-
Mechanical hardware.
-
Optical components.
-
Military weapons.
-
Hydraulic valve bodies.
-
Computer and electronic enclosures.
Type III Anodizing – Hardcoat Anodizing (or Hard Anodizing)
While hard coat anodizing is typically done in a sulfuric acid-based electrolyte, it is substantially thicker and denser than sulfuric acid anodizing. Compared to Type II, it produces a thicker (> 0.001 inches) anodized layer. Hardcoat anodize is intended for aluminum components subjected to heavy wear applications or corrosive environments requiring a thicker, harder, more lasting coating.
Additionally, it can be valuable when more excellent electrical insulation is required. Because hard coat anodize can be developed up to several thousandths in some circumstances, it is a viable option for salvaging damaged or incorrectly machined components.
Hardcoat Anodize Features:
-
Non-conductive.
-
Improve wear resistance.
-
Finishing is harder than tool steel.
-
It can be ground or lapped.
-
Can repair worn surfaces on aluminum.
-
Improve parts surface for slide applications.
-
It can be black dyed; other colors are less decorative.
Hardcoat Anodize Applications:
-
Cams
-
Gears
-
Valves
-
Pistons
-
Sliding Parts
-
Blast Shields
-
Hinge Mechanisms
-
Swivel Joints
-
Insulation Plates
Which Anodizing Process Should We Choose for the Aluminum Parts?
Considering the different applications of aluminum parts, deciding which anodizing process to use is a rather important step. So which anodized aluminum process should we choose? The following table compares the differences between Type I, Type II, and Type III.
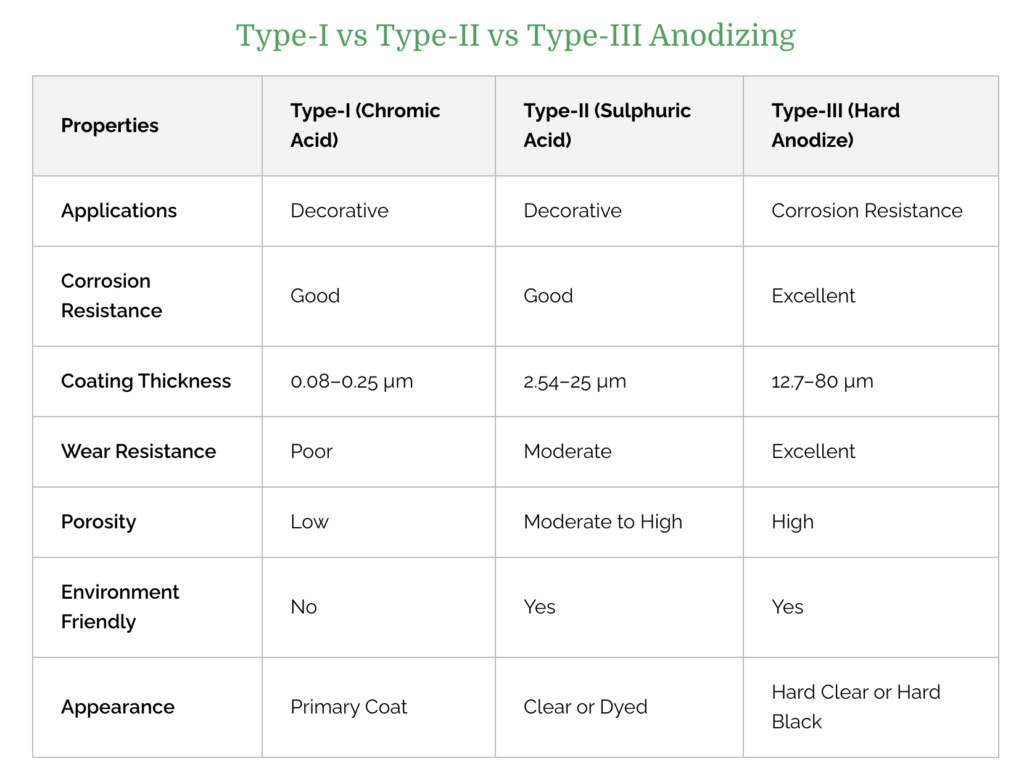
Type I: Type I applies chromic acid to the surface of metal parts to form a thin coating. While thin, chromic acid anodize offers aluminum equal corrosion protection to thicker sulfuric acid and hard coat anodize when properly sealed. So it is ideal where corrosion resistance is required, such as in manufacturing airplane parts.
Type II: Type II applies sulfuric acid to form a somewhat thicker surface layer on the aluminum. It is used to manufacture consumer goods, aerospace components, architectural parts, and kitchenware.
Type III: Type III is similar to Type II, but the resulting corrosion-resistant layer is thicker. As a result, hard coat anodize is perfectly suitable for parts that must endure extreme temperatures and chemical exposure. For example, the military uses Type III to manufacture durable metal parts.
These three types of anodized aluminum processes have their unique advantages in different applications. Choosing one depends mainly on the actual conditions of the part you need to design. If you still cannot decide which type of anodizing is more suitable for your project, SEANC is here to help you always.
How is Color Added to Anodized Aluminum Parts?
Anodized is renowned for its pigmentation. There are several methods for adding color to anodized aluminum, but the two most common are electrolytic coloring and dip coloring.
Electrolytic Coloring
Electrolytic coloring has been used to create gorgeous colors for anodized aluminum parts over the past decade. After the aluminum has been anodized, we can immerse it in another solution containing metallic salts to initiate the coloring process. Then supply the current to the anodized aluminum with pores, and the metallic salts begin to fill the base of the pores on the coating.
The ultimate pigmentation and tone of the aluminum are determined by the metallic salts used. For example, copper salts produce a reddish color. The electrolytic coloring method is extensively used because the result is resistant to ultraviolet rays and has long-lasting effects, as the salts deposit in the pores.
Dip Coloring
Dip coloring can be used to provide a wide variety of colors. Firstly, we can place the freshly anodized aluminum part in a liquid solution of organic or inorganic dyes. After the aluminum’s porous layer has absorbed the dye, the surface is bubbled in water to inhibit the further reaction. The dip coloring method offers more color options, but the ultimate result is not UV resistant and fades rapidly over time.
How do Anodized Aluminum Parts Get a Metallic Look?
Anodized aluminum has a distinct “metallic” look after coloring. Two factors cause this.
#1 When aluminum is anodized and colored, the electrochemical etching of ions in the aluminum leaves behind a rough surface. Deeper pores make the coating appear more metallic and the color more durable.
#2 Aluminum oxide’s three-dimensional crystalline structure refracts and reflects the natural light distinctively. Light strikes anodized aluminum and interacts differently with colored and uncolored metal parts. As a result of their interactions with different surfaces, the light that reaches your eye has varying wavelengths. This distinct light reflection lends anodized aluminum a distinctive metallic look that painted aluminum cannot match.
How to Seal Anodized Aluminum?
Sealing anodized aluminum is the most critical process in getting aluminum’s desired durability and improved functionality. Various methods are used to seal anodized aluminum, including high-temperature, mid-temperature, and room-temperature or cold sealing.
High-temperature Sealing
Traditionally, high-temperature sealing employs hot deionized water, with or without additives, or a high-temperature metal salt bath. This bath achieves its seal through the hydration of the anodic coating, which causes the porous layer to swell and become sealed. Hot sealing will produce results for both uncolored anodized aluminum and electrolytically colored parts. Maintaining a temperature of up to 200ºF makes this sealing method somewhat costly.
High-temperature sealing makes parts require sealing periods and high-quality deionized or distilled water. Therefore, this method makes sealing smut or bloom formation more possible. Some hot sealing bath additives permit the use of less than deionized water quality and also prevent sealing smut.
Mid-temperature Sealing
Mid-temperature sealing takes less time and can be performed at temperatures around 20-30ºF, which is lower than a hot water seal. This method provides you with energy and cost advantages over hot water sealing. Although mid-temperature sealing baths can be more challenging to control analytically than hot water, the prevention of sealing smut and uniform color appearance make them a well-balanced option.
Room-temperature or Cold Sealing
Room-temperature or cold sealing usually operates at 75-85ºF, and it has an advantage over other sealing methods because of its low energy costs and consumption. Cold sealing uses a nickel-fluoride chemical to close pores while eroding the anodic coating surface. This sealing method improves the adhesion and bonding of the anodized aluminum and decreases the likelihood of seal smut formation. Room-temperature or cold sealing is the best option if the highest quality seal is required.
What Are the Benefits of Anodized Aluminum?
Aluminum is widely utilized due to its advantageous characteristics. Although it does not rust, it is nonetheless vulnerable to other conditions. For instance, it may incur deterioration owing to oxygen exposure. Compared to standard aluminum, anodized aluminum offers several benefits, such as:
Aesthetic Appeal
Anodized metal imparts aesthetic appeal to any item. Anodized aluminum is a bright new silver hue when left natural, and it can be anodized in a matte or bright finish. You can even choose a bespoke texture such as stucco, brushed, or pebble tone to give your product a distinct look and feel.
Improve Material Properties
Aluminum is a robust material, but the anodization process makes its surface much more durable. Anodized aluminum has a three times tougher surface than standard aluminum and is impervious to chipping, flaking, and peeling, even when colored. The product will never rust, patina, or weather. Anodized aluminum is one of the market’s most durable and adaptable metals.
Safety
Because anodizing is a natural modification of the oxidation process, it is not at all hazardous to human health. It is non-toxic, does not decompose, and has high stability. Anodizing is a chemical procedure, yet it doesn’t result in any dangerous byproducts.
Design Tips for Anodizing Aluminum
It may not be challenging to learn how to anodize aluminum parts. However, following tips will make the process go more smoothly, especially if you are a beginner. Here are some valuable tips to help you.
#1 Edges And Corners
A piece of important design tip for the anodizing process is to ensure that all edges and corners of the workpiece have radii of at least 0.5 mm. Additionally, we should avoid burrs in part designs. These design considerations help prevent overheating of the workpiece caused by a high electric current concentration.
#2 Watch Out for Tolerances
If you intend to anodize your aluminum part, you should be aware that the process will add some thickness to the part because the anodizing process can impact component tolerances. Consider Type I or Type II anodizing if tight tolerances are required. You can also consider the additional layer during the design phase.
#3 Using Other Finishing Process Beforehand
Anodizing is an electrolytic passivation process. As a result, it does not produce the same results as bead blasting or polishing. If an aluminum machined part is anodized immediately, some machine marks or scratches will likely remain on the completed part’s surface. Consequently, if you need a perfectly uniform surface finish by anodizing, it may be advantageous to utilize polishing, bead blasting, or a similar mechanical finishing process beforehand.
#4 Anodize in Small Batches
Anodizing your aluminum components or products in small quantities is recommended if you are coloring them. This ensures more color uniformity, as it might be difficult to precisely match a color from one batch to the next. Color consistency is best achieved by simultaneously anodizing a small batch of small parts.
What Are the Applications for Anodizing Aluminum?
Anodizing is a cost-effective and high-quality finishing process. Therefore, it is widely used in many industries.
Medicine And Health
Anodized aluminum’s antifungal and anti-corrosive properties make it ideal for dental and medical equipment. The equipment is commonin emergency departments, operating rooms, and doctor’s offices. Medical and dental instruments require regular disinfection; hence anodized aluminum is the material of choice since it can be reprocessed again without flaking or rusting.
Food And Packaging
Anodized aluminum is perfect for the food and packaging industries because it is waterproof, easy to clean, does not flake, and is fungus resistant. In the food and packaging industries, typical uses include the manufacture of bakeware, grills, cookers, pans, kitchen equipment, and ice makers.
Electronic Products
Anodized aluminum is used to manufacture hundreds of critical electronic products with outstanding temperature resistance. In the electronic industry, anodized aluminum is utilized in computers, telephones, gaming systems, and solar panels.
Aerospace Industry
Anodized aluminum provides all of the properties required by the aerospace industry, including corrosion resistance, low density, flaking resistance, and durability in extreme environments. Even NASA employs anodized aluminum to manufacture a vast array of space equipment, such as handrails and bindings.
Conclusion
Anodized aluminum parts are extremely tough and robust. Because of its capacity to improve abrasion and corrosion resistance, this process is perfect for parts that will be used in hostile conditions. And It also offers excellent thermal insulation for metal parts. Anodizing aluminum will make metal parts more durable than untreated parts. The anodizing coating is much thinner than paints and powders but provides a much tougher surface.
Anodized aluminum can be a stunning part of your project or product, whether you’re designing impressive skyscrapers and structures, artwork, the latest line of luxury vehicles, or high-end appliances. The anodizing process is environmentally safe and delivers a finish with unrivaled beauty and durability.
SEANC has been providing high-quality anodizing services for many years. And modern plants have been built to handle low-volume projects while providing full-scale production runs. To see if anodizing is the best finishing solution for your part or product, get in touch with the SEANC team.

